Dosing
HEMLIBRA recommended dosage
The recommended dose is 3 mg/kg by subcutaneous injection once weekly for the first 4 weeks, followed by one of the maintenance dose options.
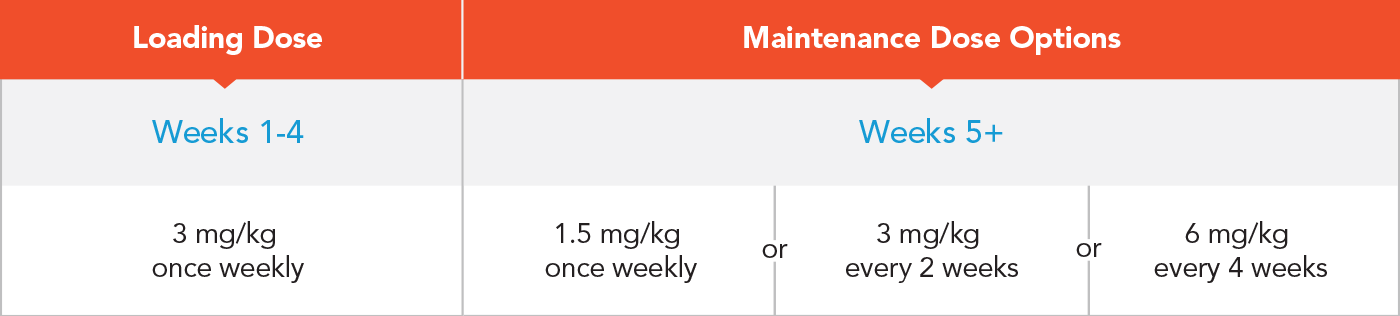
Note: Same weight-based dosage for all age ranges.
- The prophylactic use of FVIII products may be continued during the first week of HEMLIBRA prophylaxis
- Discontinue the prophylactic use of BPAs the day before starting HEMLIBRA prophylaxis
The selection of a maintenance dose should be based on healthcare provider preference with consideration of regimens that may increase patient adherence.
What are the HEMLIBRA vial strengths?
HEMLIBRA comes in four different vial strengths: 30 mg, 60 mg, 105 mg, and 150 mg. The strength and concentration are printed on the carton and vial label.
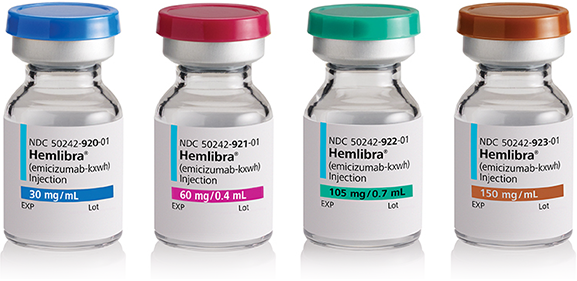
- Always check the dosage strength printed on the label to ensure you are using the correct vials.
- Your healthcare provider will tell you which vials to use to prepare the dose that will work best for you.
- Depending on your dose, you may need to use more than one vial to give your total prescribed dose.
- Refer to the HEMLIBRA Instructions for Use for handling instructions when combining vials. Do not combine HEMLIBRA vials of different concentrations in a single injection.
What should I do if I miss a dose?
- If you miss a dose of HEMLIBRA on your scheduled day, you should give the dose as soon as you remember. You must give the missed dose as soon as possible before the next scheduled dose, and then continue with your normal dosing schedule. Do not give two doses on the same day to make up for a missed dose.