What is HEMLIBRA
HEMLIBRA is a treatment for people with haemophilia A with or without factor VIII inhibitors
HEMLIBRA is a prescription medicine used for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adults and children, ages newborn and older, with haemophilia A with or without factor VIII inhibitors.
- Haemophilia A is a bleeding condition people can be born with where a missing or faulty blood clotting factor (factor VIII) prevents blood from clotting normally.
- HEMLIBRA is a therapeutic antibody that bridges clotting factors to help your blood clot.
- HEMLIBRA can reduce all bleeds (whether treated or not), and the number of treated bleeding episodes, including joint bleeds, spontaneous bleeds, and target joint bleeds.
HEMLIBRA is not a cure for haemophilia.
What is the most important information I should know about HEMLIBRA?
HEMLIBRA increases the potential for your blood to clot. Carefully follow your healthcare provider’s instructions regarding when to use an on-demand bypassing agent or factor VIII, and the dose and schedule to use for breakthrough bleed treatment. HEMLIBRA may cause serious side effects when used with activated prothrombin complex concentrate (aPCC; FEIBA®), including thrombotic microangiopathy (TMA), and blood clots (thrombotic events). If aPCC (FEIBA®) is needed, talk to your healthcare provider in case you feel you need more than 100 U/kg of aPCC (FEIBA®) total.
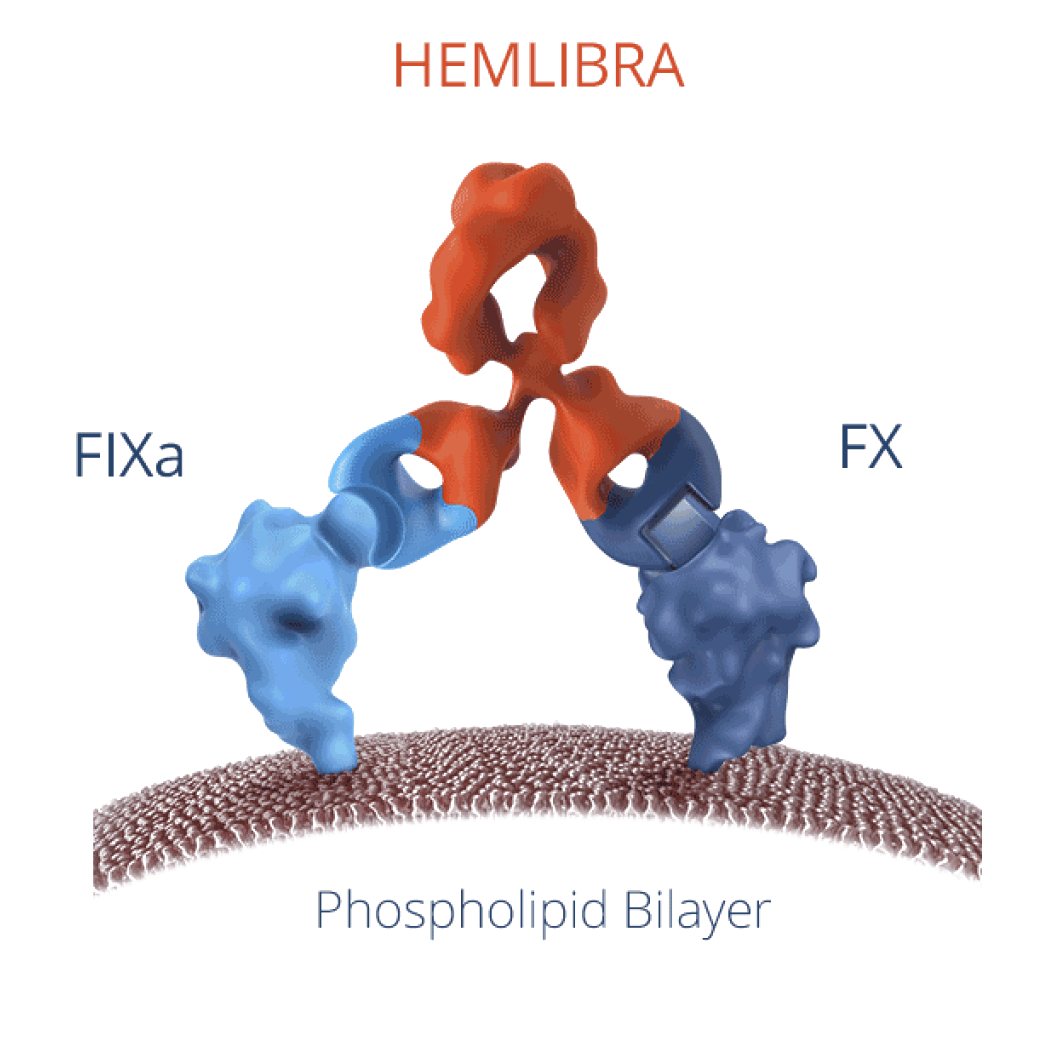
How HEMLIBRA works
HEMLIBRA is thought to bridge activated factor nine (IXa) and factor ten (x) to restore the function of missing activated factor eight (Vllla) to help your blood clot.
FIXa=activated factor IX (9); FX=factor X (10).
This material is for viewing by New Zealand audiences only
HEMLIBRA offers steady and consistent levels between doses, so there’s no need to adjust dosing based on activities
HEMLIBRA has a half-life of 4 weeks
Treatment will begin with a loading dose once weekly for 4 weeks to increase the levels of HEMLIBRA in your blood. When the loading dose is complete, you will take a maintenance dose, which helps HEMLIBRA levels in your blood remain steady. Your healthcare provider will decide how often you should take your maintenance dose, which can be once weekly (QW), once every 2 weeks (Q2W), or once every 4 weeks (Q4W).
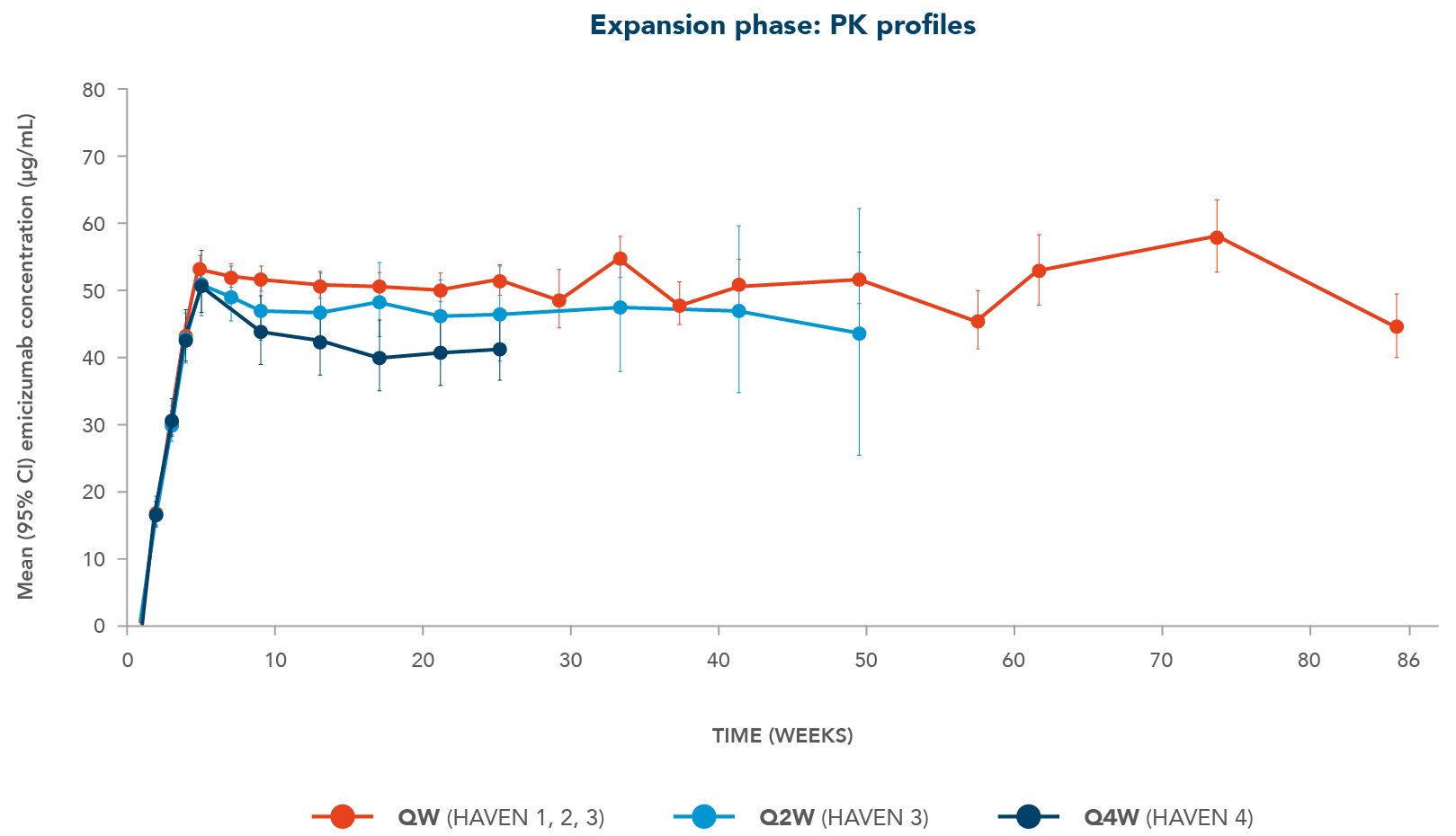
CI, confidence interval; PK, pharmacokinetics;
- Clinically efficacious concentrations obtained with all three dosing regimens (consistent with PK model predictions)
- For Q4W, emicizumab mean trough concentrations were maintained at ~41 µg/mL from Week 13 to Week 25
Adapted from Haven 1, Haven 3 and Haven 4
Lower levels of HEMLIBRA in blood were expected in children less than 6 months old.
After multiple subcutaneous injections, the amount of time estimated for the body to clear half of HEMLIBRA from the blood is 4 weeks.
Following induction dosing of 3 mg/kg once weekly for the first 4 weeks, mean trough plasma concentrations above 50 μg/mL were sustained thereafter with once weekly dosing of 1.5 mg/kg